What is left atrial appendage closure?
Some people with atrial fibrillation (AF or AFib) who are at high risk of stroke or bleed and are not able to take oral anticoagulants may require a minimally invasive procedure called Left Atrial Appendage Closure (LAAC). The LAAC procedure permanently closes off the left atrial appendage, which is known to be the main source of blood clots in patients. Closing off the LAA may reduce the risk of stroke in these patients as it prevents the migration of the blood clots to the brain.
How does left atrial appendage work?
The device is designed to close the left atrial appendage (which is known to be the main source of blood clots in patients with AFib), preventing the migration of the blood clots to the brain.
The LAAC Implant fits into your LAA. It’s designed to permanently close it off and keep those blood clots from escaping. The device is about the size of a coin and made from very light and compact materials commonly used in many other medical implants.
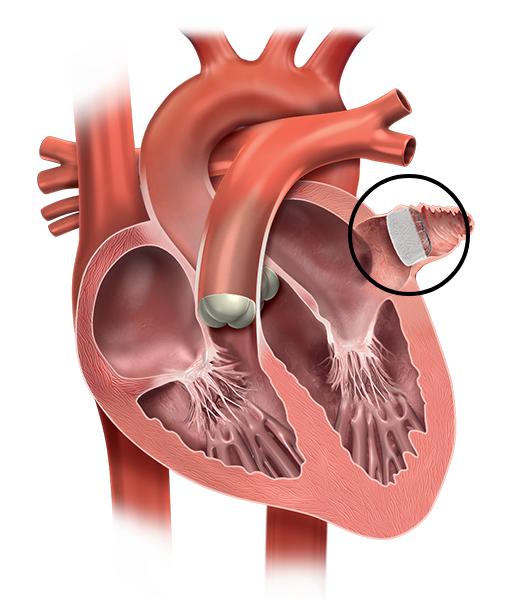
What are the benefits of left atrial appendage closure?
The LAAC is intended to prevent thrombus from escaping from the left atrial appendage and reduce the risk of life-threatening bleeding events in patients with AF not caused by heart valve problems 1,2 . If you are at risk of stroke, and should take oral anticoagulants but are unable to take them because of associated risks (high risk for bleeding included), you may benefit from the LAAC implant. Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the LAAC device.
What are the risks of left atrial appendage closure?
There are some risks associated with having a left atrial appendage closure, including:
- Stroke, clots around the device, or fluid buildup in the heart
- Damage to structures in the heart or device dislodgement
- Bleeding, chest pain, cardiac arrest, or abnormal heart rhythm
- Infection or allergic reaction to the device or medications
What is involved when having a left atrial appendage procedure?
To implant the LAAC device, your doctor makes a small cut in your upper leg and inserts a narrow tube, as done in a standard stent procedure. Your doctor then guides it into the left atrial appendage (LAA) of your heart. The procedure is done under general anesthesia or conscious sedation and takes about an hour.
Due to the risk of having a medical procedure, patients should not be considered for an LAAC device if they are doing well and expect to continue doing well on blood thinners.
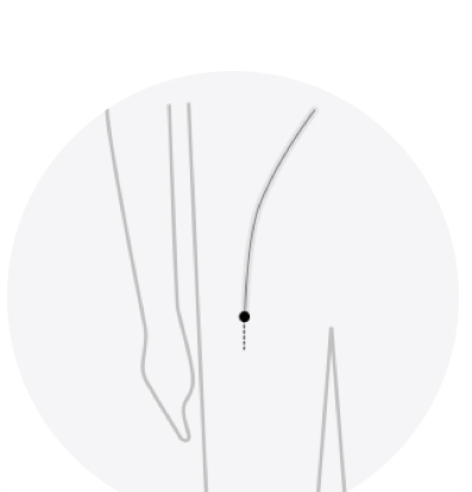 Small cut in upper leg
Small cut in upper leg
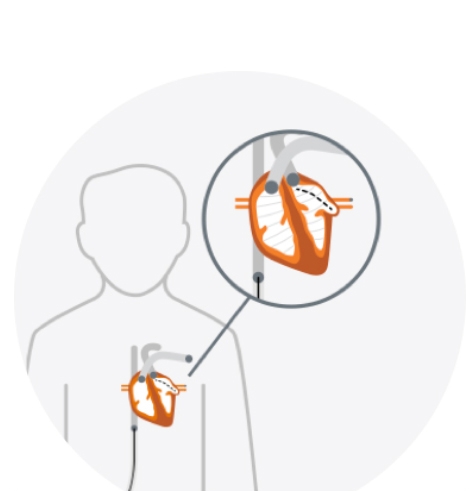 LAAC implant guided to the heart
LAAC implant guided to the heart
 Usually stay one or two days
Usually stay one or two days
 Tissue regrows, LAA sealed
Tissue regrows, LAA sealed
What happens after a left atrial appendage procedure?
After the LAAC procedure, patients usually stay in the hospital for one or two days for follow-up. Your doctor will assess your individual characteristics and conditions and will decide which is the best post implant drug regimen for you to have your LAA permanently closed off. They may prescribe you dual antiplatelet therapy (DAPT), novel oral anticoagulants (NOACs) or warfarin, along with aspirin. During this time, heart tissue will grow over the implant to form a barrier against blood clots. Your doctor will monitor this process by taking pictures of your heart to see when you can stop taking medications. Many doctors require follow up appointments over the next year to ensure your recovery is going well.
Effectiveness and outcomes following left atrial appendage closure
The success rate of LAAC varies depending on many factors such as the healing of tissue over the device in your heart, other health complications and patient characteristics. Your doctor will continue to monitor the effectiveness of the LAAC device closely by follow-up appointments and taking pictures of your heart, to see when you can stop taking medications.
There are risks associated with all medical procedures. Please talk to your doctor about the risks and benefits of a LAAC implant.
More questions about LAAC?
Learn more about a left atrial appendage closure impant in the management of atrial fibrillation.
